
Endocrine System
last authored:
last reviewed:
Introduction
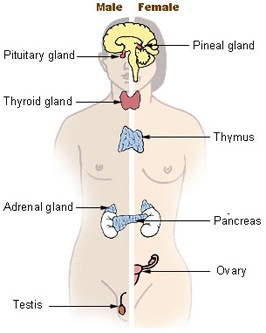
courtesy of National Cancer Institute
The endocrine system is a collection of specialized cells that are built to produce and release hormones - chemical messengers who travel around the body through blood and other fluids. Hormones cooperate and interact with the nervous system to control many of the body's functions.
Hormones can reach almost every cell in the body, except, for the most part, the central nervous system, protected by the blood-brain barrier. However, specific hormones affect specific groups of target cells, working through specific receptors.
Endocrine control tends to be slower than the nervous system, depending on blood flow, rather than nerves. At the very fastest, a hormone is secreted and works on its target in 20 seconds. Endocrine communication is highly effective for longer term control.
Many hormones are a part of feedback loops regulating homeostasis. Other hormones are active only at certain times, as occurs during human development, puberty, or the menstrual cycle.
Over 50 hormones have been discovered, with more added to the list every year. Hormones are generally classified into categories based on structure and mechanism of action.
Steroid hormones are built from cholesterol and can travel across cell membranes, as they are lipid soluble. They usually enter the cell, bind to a steroid receptor in or near the nucleus, and activate gene transcription to create new protein. They tend to be slower than non-steroid hormones.
<<PIC OF A STEROID HORMONE AT WORK>>
Nonsteroid hormones are built from amino acids, often forming polypeptide structures. They generally bind to receptors on the cell surface to open or close ion channels or activate signaling pathways inside the cell. These often activate second messengers within the cell. This is kind of like flipping a light switch outside the cell. They tend to work faster because the protein enzymes and signaling components are sitting, waiting to be act, in the cell.
<<PIC OF A NONSTEROID HORMONE AT WORK>>
In order for steroid and thyroid hormones to be soluble in blood, they require carrier proteins, ie binding globulins. Most protein hormones have binding globulins as well.
Hypothalamus
Anterior PituitaryPosterior Pituitary |
Adrenal glandThyroid
Sex Hormones
|
Homeostasis
Homeostasis is the balance of functions or levels in the body, returning biological variables to their biochemical baseline when perturbed and keeping them there.
Homeostasis uses hormones and other signals to control vital physiological processes.
These generally act in a negative feedback loop, forming a stable, self-adjusting mechanism.
<<PIC OF -VE FEEDBACK LOOP>>
Of course, feedback loops can be very complex, with the brain doing the controlling and neural and endocrine control interacting with each other.
Endocrine Tissues
Some of the body functions regulated by the endocrine system include:
- hypothalamus and pituitary
- thyroid
- parathyroid
- renin-angiotensin-aldosterone
- pancreas
- fat
- other
Hypothalamus and Pituitary
The pituitary, the master gland of the body, produces at least eight different hormones that control or affect the rest of the endocrine system. The pituitary secretes its many hormones in a pulsatile fashion, with diurnal rhythm. Positive feedback tends to come from the hypothalamus, while negative feedback comes from downstream hormonal pathways.
The pituitary is a small bean-shaped organ about 1 cm in diameter attached to the hypothalamus by the pituitary stalk.. It is located at the base of the brain, just behind the chiasm of the optic nerves and cavernous sinuses. It sits in a bony structure called the sella turcica.
The pituitary is composed of two distinct lobes - the anterior adenohypophysis (80%) and the posterior neurohypophysis.
Anterior Pituitary Hormones
Hormones from the hypothalamus controlling the anterior pituitary are transported along neural axons and released in the infundibulum, where the hypothalmo-pituitary portal vessels reside. These vessels bathe the endocrine cells of the anterior pituitary.
The anterior pituitary produces six important hormones.
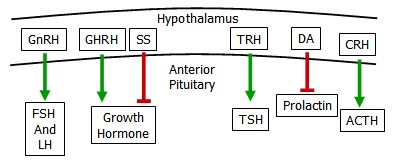
- adrenocorticotropic hormone (ACTH) released in response to stress
- thyroid stimulating hormone (TSH) controls thyroid function
- prolactin is a key mediator of lactation
- lutenizing hormone (LH) &
- follicular stimulating hormone (FSH) stimulate reproductive organs
- growth hormone is responsible for many aspects of growth
The Posterior Pituitary
Axons from the hypothalamus pass through the infundibulum to release hormones directly into blood vessels from the posterior pituitary. The two main hormones include:
- oxytocin
- anti-diuretic hormone (vasopressin)
These hormones are released from the posterior pituitary after being synthesized as large propeptides, proteolytically cleaved, and released in its active form.
Thyroid
The thyroid is an important endocrine gland located below the larynx, in front of the trachea.
The thyroid produces two main hormones. Thyroxine (T4) and its more active counterpart triidothyronine (T3) regulate body metabolism, while calcitonin decreases the rate of bone breakdown.
Parathyroid
Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) is a key regulator of blood volume. It functions in multiple parallel pathways, allowing for powerfully adjustable control.
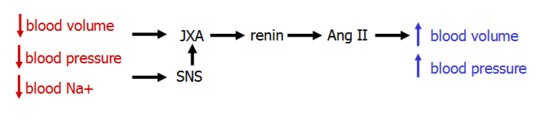
- Renin
- Angiotensin II
- Aldosterone
Renin
Renin is produced in the kidney's juxtaglomerular cells. It's secretion is stimulated by three imputs:
- norepinephrine released by symapthetic nerves in response to the systemic baroreceptor reflex
- decrease blood pressure, sensed by intrarenal baroreceptors in the JGA
- low [NaCl], sensed by the macula densa
Renin secretion is inhibited by ANP, which acts to increase GFR and sodium excretion.
Renin acts by cleaving angiotensinogen, produced in the liver, to form angiotensin I. Angiotensin I, in turn, is cleaved by angiotensin-converting enzyme (ACE) in the lung to form Ang II.
Angiotensin II
Angiotensin II is a powerful systemic vasoconstrictor that acts to increase both blood pressure and volume.
Elevated levels maintain blood pressure during hypovolemia, but can also increase blood pressure in euvolemic or hypervolemic states.
Site of Action |
Effect |
|---|---|
arterial SMCs |
vasoconstriction (NO production from bradykinin) |
adrenal gland |
aldosterone release |
SNS |
facilitates NA release |
kidney proximal tubule |
enhances Na+/H+ exchanger, leading to Na+ reabsorption |
brain |
stimulates thirst, ADH release |
heart |
inc. contractility and ventricular hypertrophy |
While Ang II constricts both afferent and efferent arterioles of the glomerulus, its dominant effect is efferent constriction.
This increases GFR and filtration fraction in the face of decreased renal blood flow is due to increased capillary osmotic pressure and decreased hydrostatic pressure.
Also, decreased vasa recta blood flow decreases urea washout from the medulllary interstitium, increasing the gradient for NaCl reabsorption in the thin ascending loop of Henle. This increases Na reabsorption.
The net effect is water and sodium retention.
Molecular Mechanisms of Action
The AT1 receptor is a GPCR that can induce many different cell signaling pathways. Akin to the binding of epinephrine to α2 receptors, AT1 activation in the proximal tubule inhibits adenylyl cyclase and cAMP production, which in turn increases the activity of the Na/H exchanger.
In the vasculature, activation of AT1 mimics epinephrine binding to α1 receptors by increasing levels of DAG and IP3.
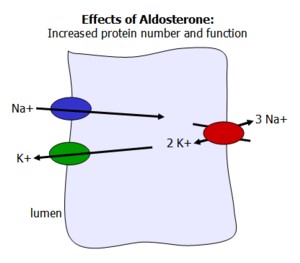
Aldosterone
Aldosterone is a mineralcorticosteroid hormone synthesized from cholesterol in the adrenal cortex. It is active during times of volume contraction and low sodium concentration, working to increase plasma sodium levels, and thereby water.
While aldosterone's effects on sodium reabsorption are most prevalent in the principal cells of the distal tubule and collecting duct in the kidney, it also works on sweat glands and the colon to increase sodium uptake.
Aldosterone secretion is regulated in a number of ways:
- Ang II activity
- hypokalemia, sensed by the zona glomerulosa in the adrenal cortex
- ANP reduces aldosterone secretion
Aldosterone also works on muscle during times of hyperkalemia to increase Na/K pump activity to decrease ECF [K]. Increased K secretion in the cortical collecting tubule also reduces K concentration.
Intracellular Signaling of Aldosterone
Aldosterone binds to a cytoplasmic receptor and migrates to the nucleus, where it activates transcription of protein channels and pumps. It also activates proteins that increase existing channel and pump function, resulting in increased sodium reabsorption and potassium secretion. This includes apical Na and K channels, and the basal Na/K pump.
Aldosterone is involved with acid secretion, and icnreased aldosterone can cause metabolic alkalosis. Not sure how.
Loss of Aldosterone
Loss of adrenal function, as occurs in Addison's Disease, results in severe sodium depletion, ECF volume contraction, and circulatory insufficiency.
Pancreas
The pancreas is an important endocrine gland, secreting hormones that regulate blood glucose levels and cellular metabolism across the body.
It is also an exocrine gland, producing enzymes responsible for digestion in the small intestine.
Anatomy and Histology
The pancreas is located in the retroperitoneum weighs 70-120g, and is 12-20 cm in length. The head of the pancreas lies in the C loop of the duodenum, while its tail lies behind the stomach and points towards the spleen.
Endocrine functions
The pancreas is composed of 1-2% islet cells. These produce important hormones such as insulin, glucagon, somatostatin, and pancreatic polypeptide.
The pancreas consists of about 1 million clusters of cells call the islets of Langerhans. Each is roughly 100-200 um and consists of four major cell types:
- beta (68%) - produce insulin
- alpha (20%) - produce glucagon
- delta (10% - produce somatostatin
- PP (Pancreatic polypeptide) cells (2%) - produce pancreatic polypeptide
- D1 cells - vasoactive intestinal polypeptide, VIP,
- enterochromaffin cells - serotonin
The Pancreas and Digestion
The pancreas is comprised of 95% acinar cells and 1-2% islet cells. Acinar cells form exocrine glands, producing proteolytic enzymes which are released into pancreatic ducts and flow into the duodenum through the amuplla of Vater. Acinar cells also secrete protective enzymes such as pro-colipase and trypsin inhibitor. Acinal cell secretion is controlled by CCK. Lipase, amylase, and ribonuclease are all secreted in active form, while proteolytic enzymes are inactive until converted by trypsin.
Ductal cells secrete water, electrolytes, and bicarbonate, decreasing secretion viscosity and alkalinizing (3.5-4) the intestinal environment to enhance enzyme activity. 1-2 lites of fluid are secreted into the small intestine daily. Duct cell secretion is controlled by secretin.
Pancreatic secretion of lipid digesting enzymes is controlled by hormonal signals from the duodenum and jejeunum, including cholesystokinin (CCK). CCK acts both on the gall bladder and pancreas and also decreases gastric motility.
Intestinal cells also produce secretin, causing the pancreas and liver to produce bicarbonate and thereby increase the pH.
Acinar cells secrete digestive enzymes. Proteolytic enzymes are secreted as inactive zymogens, but others, such as alpha-amylase and lipases, are secreted as functional enzymes. Acinar cells also secrete protective enzymes such as pro-colipase and trypsin inhibitor. Acinal cell secretion is controlled by CCK.
Pancreatic duct cells secrete bicarbonate, water, and electrolytes. Duct cell secretion is controlled by secretin.
Fat
Intra-Abdominal Obesity
IAA can be measured using waist circumference, which is strongly correlated with CT/MRI (the gold standard)
Adipokines are powerful hormones released by adipose tissue. The more fat, the stronger the signal. Adipokines include leptin, releasing, and others. They are responsible for type II diabetes.
High visceral fat correlates with elevated TG and low HDL-C.
Fat Growth
Adipocyte energy intake comes primarily from two sources - glucose and lipids.
Effects of insulin on fat are:
Carbohydrate Metabolism
- Increased glucose uptake, through GLUT-4 transporters,
- Increased glycolysis and production of DHAP and glycerol 3-phosphate
- Increased activity of the pentose phosphate pathway and subsequent NADPH production
Lipid Metabolism
- Increased FFA absorption from chylomicrons and VLDL through action of lipoprotein lipase
- Increased TAG synthesis from a high insulin/glycogen ratio
- Decreased TAG degradation
Excess caloric intake over time can lead to changes in adipocyte enzymes and obesity.
Shrinking Fat
As blood sugar (and insulin) levels fall, the liver begins breaking down glycogen in a process called glycogenolysis to liberate glucose. As these stores run out within a day or two, and blood sugar begins to depend on fat release.
Fat responds to a high epinephrine/insulin ratio by activating hormone-sensitive lipase (via cAMP and PKA) to chop triacylglycerols (TAGs) in glycerol and FFAs, which are released into the blood.
FFAs are transported on albumin and taken up by various tissues, while glycerol is returned to the liver for TAG synthesis or gluconeogenesis.
Decreased lipoprotein lipase activity leads to decreased FFA uptake.
Mitochondrial Uncoupling
Chemical uncouplers, including dinitrophenol and salicylate (aspirin), are lipid-soluble compounds with a pKa near 7.2.
Uncoupling proteins include UCP1-5, and are found in various tissues.
Uncouplers enter the inner mitochondria and induce proton leak into the mitochondrial matrix without generating ATP. This energy is instead released as heat.
Uncoupling occurs in brown adipose tissue in response to norepiniephrine stimulation, triggering heat generation.
Other
These important endocrine tissues and functions are described elsewhere:
- the menstrual cycle
- sperm and testosterone production
- lactation
- growth
- anti-diuretic hormone and fluid balance
- liver - processor, storager, and isposer of nutrients and wastes
