
Biology Template
last authored:
last reviewed:
Introduction
courtesy of National Cancer Inst.
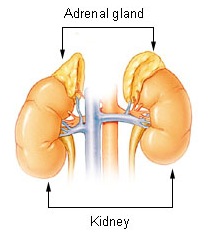
The adrenal gands are small (~4g) yellowish-coloured organs located above the kidney. They are located retroperitoneally.
The adrenal glands are responsible for the production of various hormones of the body.
Anatomy
The adrenal glands are divided into the cortex and the medulla.
Regulation
Adrenocorticotropin Hormone (ACTH) is a hormone released from the anterior pituitary. It acts on the adrenal cortex, where it induces expression of hormones called glucocorticoids, including cortisol. ACTH release is mediated by G protein signalling and calcium influx after signalling by Corticotropin-Releasing Hormone (CRH). This is a stress responsive hormone released by the hypothalamus in response to a number of factors, including:
- low cortisol
- hypoglycemia
- stress, anxiety, and depression
- trauma
- pyrogens
- various neurotransmitters
Cortisol provides negative feedback to the hypothalamus and pituitary.
Normally, cortisol expression is highest at night and is secreted in pulses. The unstressed adult produces 10-20 mg daily.
Adrenal Cortex
The adrenal cortex produces steroid hormones, including
- aldosterone (zona glomerulosa)
- glucocorticoids (zona fasciculata)
- DHEA and other androgens (zona reticularis)
Aldosterone is primarily regulated by the renin-angiotensin II-aldosterone system, while cortisol and androgens are largely regulated by ACTH from the anterior pituitary.
<<PIC of STEROID HORMONES AND PRODUCTION>>
Cortisol
Glucocorticoids are steroid hormones produced by the adrenal cortex. The most important glucocorticoid is cortisol.
Cortisol is involved in stress-related conditions and glucose metabolism. Cortisol is bound to corticosteroid binding globulin (CBG - 75%) and albumin (15%) in the blood. CBG levels increase during times of high estrogen, hyperthyroidism, diabetes, and some genetic conditions. Levels can decrease with cirrhosis, nephrotic syndrome, hypothyroidism, and familial CBG deficiency.
Cortisol is metabolized mainly in the liver, along with most other adrenal steroids. After conjugation with glucuronic acid it is excreted in the urine.
Regulation of Blood Glucose
Glucocorticoids act to increase and maintain normal blood glucose levels during periods of stress by increasing glycogenolysis and gluconeogenesis in the liver, increasing amino acid and fatty acid liberation from muscle and adipose tissue, respectively, to provide substrates for gluconeogenesis, and by decreasing uptake and utilization of glucose in muscle and adipose tissue.
Regluation of the immune response
Glucocorticoids are potent anti-inflammatory and immunosuppressant molecules, acting in part by inhibiting IL-1β, phospholipase A2, COX-2, leading to decreased arachidonic acid and prostaglandin production. They also lead to decreased local production of histamine and bradykinin.
Other Effects
Glucocorticoids also act on lymphoid, skin and connective, adipose, muscle, and hepatic tissue. They inhibit edema by potentiating vascocnstriction caused by norepinephrine and epinephrine. They inhibit fibroblast growth and reduces ECM deposition, reducing scarring, and also decrease circulating lymphocyte levels, mainly by inducing redistribution to other compartments.
Glucocorticoids are transported across the plasma membrane and signal through glucocorticoid receptor, which is present as two isoforms GRα and GRβ. GRα can both activate or inhibit gene transcription, while GRβ acts as a dominant-negative inhibitor of GRα.
GCs block transcription by NFkB, inhibiting the expression of IL-1β, IL-2, and TNF. This blocks IL-2 and IFNγ expression, leading to a decrease in leukocyte activity
Ten percent of free cortisol is biologically active; 75% is bound to cortisol-binding globulin (CBG) and 15% is bound to albumin.
Cortisol is metabolized in the liver by reduction and conjugated with sulfate or glucuronate, and excreted in the urine.
Because cortisol can bind mineralcorticoid receptor in the kidney and cause inappropriate activation of aldosterone-related pathways, a CP450 enzyme called 11βHSD2 converts cortisol to inactive cortisone in the kidney. Cortisone is then transported to sites of action, including the skin, liver, adipose tissue, and muscle, where 11βHSD1 reconverts cortisone into active cortisol.
Severe Cushing's disease results in levels of cortisol that override the capacity of 11βHSD2, leading to hypertension and hypokalemia. Glycyrrhic acid in licorice inhibits 11βHSD2, leading to similar symptoms.
GC overproduction can do many bad things.
Aldosterone
Mineralcorticoids describe aldosterone and 11-deoxycorticosterone (DOC)
Aldosterone is produced by the zona glomeroulosa of the adrenal cortex and regulated sodium and potassium levels.
Binding to MR in the epithelium of renal distal and collecting tubules induces altered gene expression and activit yof ion channels. As a result, sodium, bicarbonate, and water are reabsorbed, while potassium is excreted.
Metabolism
Aldosterone has a half-life of 15-20 minutes and is metabolized in the liver and kidney.
Free plasma aldosterone levels are 3-15 ng/ml, representing 30-50% of total aldosterone levels.
Dehydroepiandrosterone (DHEA)
DHEA is a steroid precursor of androgens and estrogens.
DHEA is produced in small amounts by the adrenal cortex (zona reticularis). In males it has little effect due to the large amounts of testosterone present.
In females, DHEA is responsible for many of the same effects as testosterone, including enhancement of female pubertal growth, development of axillary and pubic hair, and development and maintenance of female sex drive.
Adrenal insufficiency can cause pubic hair loss in females due to loss of DHEA production. Testosterone production by the testes spares men from prominent effects of DHEA loss.
Adrenal Medulla
The adrenal medulla produces catecholamines.
Dopamine is the precursor of norepinephrine, a sympathetic neurotransmitter. The medulla is almost the exclusive site of the converting enzyme used to generate epinephrine, a key stress hormone.
Medullary secretions are about 85% epinephrine and 15% norepinephrine.
The medulla contains sympathetic ganglion cells and chromaffin cells, also known as pheochromocytes. These cells migrate from the neural crest during development, and it is possible some could be left behind along the aorta, the iliac arteries, and rarely in the bladder. These sites can also birth phaeochromocytomas.
Epinephrine is the primary 'fight-or-flight' hormone and works together with norepinephrine to induce its spectacular responses. It is secreted from the adrenal medulla in response to hypoglycemia, stress, trauma, or extreme exercise. Its release is largely controlled by the CNS via preganglionic sympathetic fibres.
Epinephrine works to increase cardiovascular function and rapidly mobilize energy-yielding fuels, working in a variety of mechanisms:
- increased heart rate and contractility (β1 receptors to increase heart rate (IF), conduction rate (ICa)
- arteriolar constriction through α1 receptors
- venous contstriction to increase venous return
- decreases renal GFR
- increases Na-H exchanger and Na-K pump in the proximal tubule, increasing Na reabsorption via α2 receptors and inhibition of adenylate cyclase/cAMP. This is akin to the effetcs of Ang II.
- inhibits insulin secretion by pancreatic beta cells to keep blood glucose levels elevated
- increases glycogenolysis and gluconeogenesis in muscle (and liver?)
- increases plasma levels of free fatty acids and DAG through action of hormone-sensitive lipase in adipose tissues
Adrenergic Receptors
Generally, the various adrenoreceptors can bind both epinephrine and norepinephrine, though with different affinities.
Intracellular Effect |
location |
Physiologic Effect |
|
|---|---|---|---|
α1 |
inc IP3 and DAG lead to inc Ca2+ |
|
arteriolar vasoconstriction by SMCs |
α2 |
dec [cAMP] |
|
vasoconstriction in some vessels, Na uptake in the proximal tubule |
β1 |
inc [cAMP] |
heart |
increases heart rate and contractility |
β2 |
inc [cAMP] |
arterioles |
vasodilation |
β3 |
inc [cAMP] |
|
|
The biologic effects of the catecholamines are very brief, lasting for only 10 seconds in the case of epinephrine. Enzymes including COMT and MOA convert it to vannilylmandelic acid (VMA), which can be measured in the urine.
Function
important conditions and diseases
Cell Biology
Development
Resources and References
Topic Development
authors:
reviewers:
