
Carbohydrates
last authored:
last reviewed:
Introduction
Carbohydrates range from simple sugars, such as glucose, to large and complex trees made out of simple sugars.
Types of Carbohydrates
- monosaccharides
- polysaccharides
- fibre
- Tab 5
- glycogen
- sugar alcohols
- glycosaminoglycans
Monosaccharides
Lactose
- major carbohydrate of breast milk
- lactose is a 6-sided sugar
- produced in mammary epithelial cells' Golgi Apparatus of by lactose synthetase, composed of lactalbumin and galactosyl transferase, both from ER
Polysaccharides
Fibre
aim for 27-40 grams/day
Insoluable fibre
- cellulose, lignin, and some hemicellulose
- guards against constipation
Soluble fibre
- pectins in fruits
- gums, mucilage, some hemicellulose
- helps bind cholesterol
Cell surface carbohydrates
Sialic acid is a generic term for the N- or O-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone. It is also the name for the most common member of this group, N-acetylneuraminic acid (Neu5Ac or NANA). Sialic acids are found widely distributed in animal tissues and in bacteria, especially in glycoproteins and gangliosides.
Sialic acid acts as the adhesion molecule for influenza virus protein hemagglutinin.
Glycogen
glycogen binds a lot of water.
Glycogen is a highly branched polysaccharide made exclusively from alpha-D-glucose that can weigh up to 106 kDa. It exists in discrete cytoplasmic granules that contain most of the enzymes required for its synthesis and degradation.
About 400 g of glycogen make up 1-2% of the fresh weight of muscle, and about 100 g of glucogen make up 10% of the weight of a well-fed adult liver.
Liver stores of glycogen increase following meals and decrease during fasting, becoming depleted within 12-24h. Muscle stores of glycogen are only moderately affected by prolonged fasts and are replenished following depletion due to strenous exercise.
Regulation of glycogen synthesis and degradation occurs on two levels.
Allosteric control allows response to metabolic and energy needs of the cell.
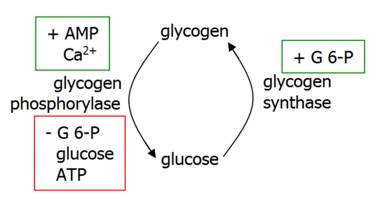
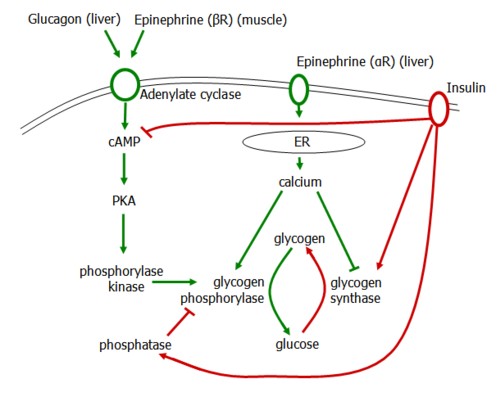
Glycogen degradation is accelerated by the hormones glucagon in the liver and epinephrine in muscle (beta receptors) and liver (alpha receptors).
Glucagon and beta receptors activate adenylate cyclase, generating cAMP and activating PKA. This activates phosphorylase kinase, which then activates glycogen phosphorylase to induce glycogenolysis.
Alpha receptors release calcium stores from the ER, activating Ca-dependent calmodulin that activates PKA as above and also inhibits glycogen synthase.
Glycogen Synthesis is induced by insulin, which degrades cAMP, activates glycogen synthase, and inhibits glycogen phosphorylase.
Glycosaminoglycans
Because of their large number of negative charges, GAGs become surrounded by a shell of water. They repel each other, and when brought together they slip past each other, as do two negative poles of magnets. This makes GAG-containing fluids, such as mucous secretions and synovial fluid, slippery.
When a GAG-containing solution is compressed, water is squezzed out. When compression is released, the many negative changes on the GAGs repel each other and cause the tissue to spring back to its initial position.
Glycosaminoglycan Locations
GAGs are found in a variety of tissues....
Carbohydrate Absorption

Glucose is transported into intestinal and renal cells against its concentration gradient, thus requiring energy provided by the co-transport of sodium along its concentration gradient.
Glucose Transporters
There are 14 tissue-specific members of the glucose transport family.
Transporters in the brain, liver and RBCs are expressed at high levels to allow rapid entry of glucose.
Cell surface expression of transporters in skeletal muscle and adipose tissue (Glut 4) are controlled by insulin.
Transporter |
Distribution |
Properties |
|---|---|---|
Glut 1 |
RBCs, brain capillaries, kidney |
may limit glucose in brain |
Glut 2 |
liver, pancreas beta cells, basal surface of SI |
high capacity, low affinity |
Glut 3 |
neurons, placenta, testes |
low Km |
Glut 4 |
adipocytes, muscle |
insulin-mediated |
Glut 5 |
SI, testes, sperm |
fructose transport |
Glut 7 |
liver and glucogenogenic tissues |
glucose transport across ER |
Control of Glucose Entry
The blood brain barrier allows glucose to be taken up exclusively through capillaries due to tight junctions, narrow intercellular space, and a continuous basement membrane. Other non-neural tissues sometimes permit glucose to be taken up between cells, and also by pinocytosis.
Glucose entry into cells can be controlled at the cell surface by insulin, as is the case of Glut-4 in adipocytes and muscle (skeletal and cardiac).
Insulin can also affect glucose metabolism by affecting glucokinase transcription.
Carbohydrate Metabolism
- glycolysis
- gluconeogenesis
- glycogenesis
- glycogenolysis
- electron
transport chain - TCA
cycle - pentose phosphate
pathway
Glycolysis
The ten reactions of glycolysis, or the breakdown of glucose into pyruvate, occurs in two stages.
The first five reactions correspond to an energy investment of two ATP, while the last five produce 4 ATP for a net amount of 2 ATP per glucose. The reactions of Hexokinase, PFK-1, and pyruvate kinase are irreversible.

Phosphorylation of glucose to glucose-6-phosphate occurs through the action of various isoforms of hexokinase, which has a low Km (and therefore high affinity) for glucose. This also means that it has a low VMax, however.
In the liver and pancreatic islet cells, glucokinase is the predominant enzyme that phosphorylates glucose. Glucokinase has a high Km and VMax, preventing it from working at low glucouse concentrations but allowing it to respond to a flood of glucose, minimizing systemic hyperglycemia.
Phosphorylation of fructose-6-phosphate to fructose-1,6-bis P is irreversible and catalyzed by phosphofructokinase-1 (PFK-1). This rate-limiting step is the most important control point of glycolysis.
Cleavage of fructose 1,6-bisphosphate yields a molecule each of glyceraldehyde-3-P and DHAP, which is converted into glyceraldehyde-3-P.
Oxidation of glyceraldehyde-3-phosphate results in 1,3-BPG, which can be converted to 2,3-BPG, an allosteric controller of hemoglobin affinity for oxygen.
A few other steps occur to produce phosphoenolpyruvate (PEP).
PEP is converted to pyruvate through the irreversible action of pyruvate kinase, generating ATP.
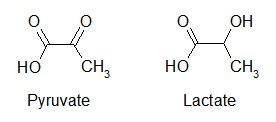
Pyruvate can then go on to enter the TCA Cycle, or can by converted into lactate.
Lactate
Reduction of pyruvate to lactate, but lactate dehydrogenase, is the last step of anaerobic glycolysis. Lactate is the major fate of pyruvate in RBCs, the eye, the kidney medulla, testes, and leukocytes. RBCs produce the most lactate per day (30g) followed by skin, brain, skeletal muscle, and renal medulla.
An elevated NADH/NAD+ ratio in skeletal muscle can occur during intense exercise due to increased activity of glyceraldehyde 3-P dehydrogenase and production of NADH that exceeds the capacity of the TCA cycle. This increased NADH favors lactate production, leading to a drop in pH and increased release of oxygen in muscles. Much of this lactate diffuses into the blood and can be used in the liver for gluconeogenesis.
Elevated lactate levels are present in the plasma during circulatory collapse, as can occur during an MI, a PE, uncontrolled hemmorage, or shock. Execess oxygen is required to require from a time of low oxygen, and this amount is called the oxygen debt. The level of oxygen debt has been related to morbity and mortality.
Regulation of Glycolysis
In the liver, glucokinase is normally sequestered on the nucleus. Increasing intracellular glucose facilitates release of glucokinase into the cytoplasm. As glucose levels fall, fructose-6-phosphate causes glucokinase to translocate back to the nucleus and become inactive. Glucokinase transcription is increased by insulin.
PFK-1 is activated by fructose 2,6-bisphosphate, which is synthesized by PFK-2. F-2,6-bisP is increased by a high insulin/glucagon ratio, activating PKA and PFK-2. It is also stimulated by AMP.
In cardiac muscle, norephinephrine activates PFK-2, while in skeletal muscle, fructose-6-P activates it. In liver, a high insulin/glucagon ratio activates PFK-2, which a low ratio inhibits it.
PFK-1 is inhibited by high levels of ATP and citrate, an intermediate in the TCA cycle. The levels of ATP in cardiac muscle would inhibit PFK-1 were it not for the presence of AMP and F-2,6-bisP.
F-2,6-bisP also acts to inhibit fructose 1,6-bisphosphatase in the gluconeogenesis pathway. This prevents both pathways from being active at the same time.
Gluconeogenesis
Gluconeogenesis is used as a source of blood glucose during the fasting state, peaking around two days. Levels begin to rise 4 hours after.
While all tissues use glycolysis as an oxidative source of energy, the liver and kidney are the major sites of gluconeogenesis. Of the glucose produced, 80% comes from the liver and 20% from the kidney. Kidney gluconeogenesis is coupled to ammonia production.
From Pyruvate to Glucose
Pyruvate, residing in the mitochondria, cannot be transported across the mitochondrial membrane itself. Instead, it is carboxylated to OAA, which is temporarily converted to malate for transport across the mitochondrial membrane. Once in the cytosol, malate becomes OAA again, which is then converted to phosphoenolpuruvate (PEP) in the glycolytic pathway.
All glycolytic reactions are reversible save three, which require specific enzymes. Glucose 6-phosphatase is located only in the liver.
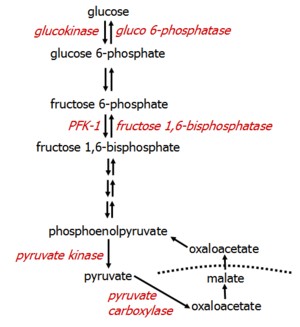
Beyond molecules of the TCA cycle, the most important molecules that enter gluconeogenesis include glycerol, lactate, and α-ketoacids.
Glycerol
Glycerol is released during TAG hydrolysis in adipose tissue and delivered to the liver.
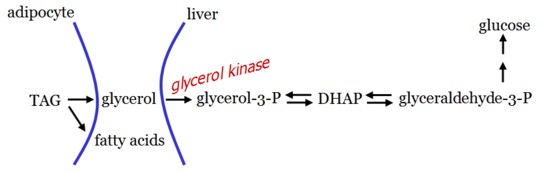
As adipocytes cannot phosphorylate glycerol, gluconeogenesis from glycerol can only occur in the liver.
Lactate
Lactate enters the blood from cells lacking mitochondria, such as RBCs, or by exercising muscle. Lactate returns to the liver, where it is reconverted to glucose for re-release into the blood in the Cori Cycle.
Protein-Dependent Gluconeogenesis
Urine excretion of urea is low during the fed state but increases dramatically 12 h after fasting, decreasing to fed state levels after a few weeks, providing a measure of increased protein degradation.
Amino acids can enter the TCA cycle at various points, including alpha-ketoglutarate, succinyl CoA, fumarate, and oxaloacetate. They can also be converted into puruvate and acetyl CoA. From there, oxaloacetate can be converted into PEP and returned to glucose.
Control of Gluconeogenesis
The ratio of insulin:glucagon regulates both glycolysis and gluconeogenesis.
Fructose 1,6-bisphosphatase is an important control point for gluconeogensis, and inhibited by high levels of fructose 2,6-bisphosphate, a potent activator of PFK-1 in glycolysis.
The insulin/glucagon ratio plays an important role in mediating F 2,6-bisP levels.
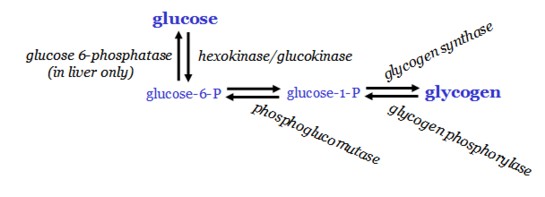
Glycogenesis
Glycogenesis occurs in the absorptive state, when blood glucose levels are high. It is activated by a high insulin:glycogen ratio and the availability of glucose 6-phosphate.
Glycogen is synthesized in the cytosol in a process that requires ATP and UTP. Two different bonds are formed - alpha 1-4 and alpha 1-6.
Glucose 6-phosphate is converted to glucose 1-phosphate by phosphoglucomutase, and G 1-P is attached to a UDP molecule and bound to a protein glycogen primer, glycogenin, by glycogen synthase. Further elongation occurs by glycogen synthase, and a branching enzyme forms alpha 1-6 linkages (instead of alpha 1-4) every 10 residues or so. These branches greatly accelerate synthesis and degaradation times of glycogen and its size as well.
Glycogenolysis
Glycogenolysis, the breakdown of glycogen into monomeric glucose, occurs during times of increased energy demand in the liver and muscle, the major stores of glycogen.
Glycogenolysis is the return of polymeric to monomeric glucose. Cleavage of glycogen by glycogen phosphorylase yields glucose 1-phosphate, which is converted back into glucose-6-phosphate by phosphoglucomutase.
Glucose-6-phosphate cannot exit the cell, and so, in the liver, glucose 6-phosphate is transported into the endoplasmic reticulum, where it is converted into glucose by glucose 6-phosphatase. Glucose then exits the cell through the Glut-2 transporter. Muscle lacks glucose-6-phosphatase and therefore cannot contribute to maintenance of blood glucose levels. Instead, it is used as a rapidly mobilizable source of oxidizable glucose for ATP production.
Cleavage of glycogen by glycogen phosphorylase yields glucose 1-phosphate, which is converted into glucose-6-phosphate by phosphoglucomutase.
Muscle Use of Glycogen
Muscle use of glycogen as fuel occurs immediately after onset of exercise and last for about an hour.
Glycogenolysis is induced by three factors in muscle:
- Nerve impulses that activate muscle contraction act to release calcium stores from the sarcoplasmic reticulum. Ca2+-calmodulin increases glycogen phosphorylase
- Epinephrine binding to its cell surface receptor activate the AC/cAMP/PKA pathway, inducing glycogen phosphorylase
- Muscle contraction uses ATP, and production of AMP from ADP is another activator of glycogen phosphorylase
In liver, glycogenolyis is positively regulated by glucagon and epinephrine and negatively regulated by insulin.

Glycogenolysis can also be controlled by allosteric regulators.

Electron transport chain
Cellular respiration is the metabolism of carbohydrates and other molecules, using oxygen, and generating carbon dioxide during ATP synthesis.
The electron transport chain (ETC) occurs in the mitochondrion following generation of NADH and FADH2 from the TCA cycle. These coenzymes, when oxidized, can donate a pair of electrons to a set of specialized electron carriers.
The ETC is present in the inner mitochondrial membrane and is occurring continuously in all tissues containing mitochondria.
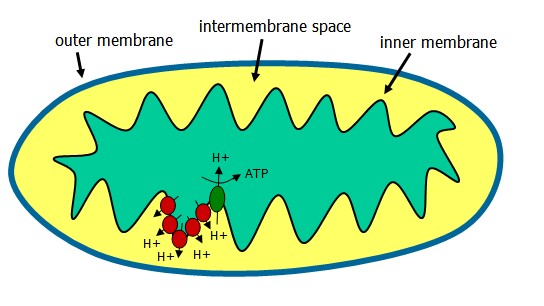
NADH is reduced by dehydrogenases that remove two hydrogens. Both electrons and one proton (a hydride ion) are transferred to the NAD+, forming NADH plus H+. The proton and hydride carried by NADH are next transferred to NADH dehydrogenase, complex 1 in the inner membrane. Coenzyme Q is complex II, and electrons are passed along the chain from complex II to the cytochromes of complex III and IV. Cytochromes, like hemoglobin, contain a heme group made up of a porphyrin ring and an atom of iron. The cytochrome iron is reversibly converted from its ferric (3+) to ferrous (2+) form as a normal part of its function.
Oxygen, an avid electron acceptor, is at the end of the electron transport chain. O2 + 4H+ -> 2 H20.
The TCA Cycle
The Tricarboxylic acid cycle, also called the Krebs cycle or the citric acid cycle, is the final pathway where oxidative metabolism of carbohydrates, amino acids, and fatty acids converge. Carbon skeletons are converted into carbon dioxide and water, producing energy for the production of the majority of ATP.
The TCA cycle occurs in the mitochondria in close proximity to the electron trnasport chain.
The TCA cycle may be viewed as a traffic circle, with components entering and leaving at various points as required.
Reactions of the TCA Cycle
Puruvate, the final product of aerobic glycolysis, is transported into the mitochondrion matrix by a specific transporter and irreversibly converted into acetyl CoA by a multi-enzyme complex.
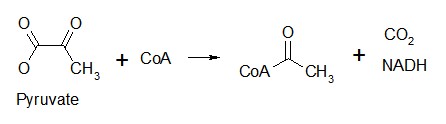
Five coenzymes are required for this process: thiamine pyrophosphate, lipoic acid, coenzyme A, FAD, and NAD+. Arsenic poisoning inhibits enzymes requiring lipoic acid as a cofactor.
Acetyl CoA enters the TCA cycle and combines with oxaloacetate to form citrate, which provides negative feedback to glycolysis by inhibiting PFK-1.
The sequence of reactions then goes: citrate, isocitrate,α-ketoglutarate, succinyl CoA, succinate, fumarate, malate, oxaloacetate, and finally back to citrate.
A,C,I,K,SC,S,F,M,O
Energy Produced by the TCA Cycle
Two carbon atoms enter the TCA cycle as Acetyl CoA and leave as CO2. During one turn of this cycle, three pairs of electrons reduce NAD+ to NADH, one pair reduces FAD to FADH2, and one GTP is produced. NADH and FADH2 then go on to the electron transport chain, where they are used to generate ATP. Oxidation of one NADH results in two ATP, while one FADH2 yields two ATP.
| Reaction | # of ATP |
|---|---|
| 3 NADH | 9 |
| FADH2 | 2 |
| GTP | 1 |
| Net: | 12 |
Exit of Metabolites in the TCA Cycle
Various components of the TCA cycle can be used as substrates for other metabolic pathways.
Pyruvate can also enter the TCA cycle through conversion by puruvate carboxylase to oxaloacetate, providing more carbon to the system if it is actively being used to synthesize proteins or lipids.
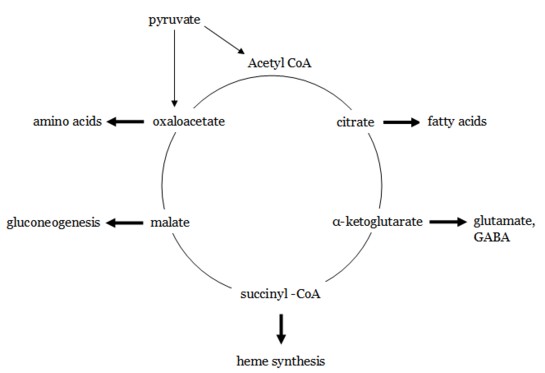
Entry Into the TCA Cycle
The Pentose Phosphate Pathway
The pentose phosphate pathway (PPP), also called the hexose monophosphate shunt, occurs in the cytosol. It is a major source of the body's NADPH, a biochemical reductant. It also produces ribose 5-phosphate, used in the synthesis of nucleotides.
Glucose 6-phosphate, the first metabolite of glycolysis, enters the PPP, producing one molecule of C02 and 2 molecules of NADPH.
The PPP consists of two irreversible oxidative reactions followed by a series of reversible interconversions. No ATP is directly consumed or generated.
Irreversible Oxidative Reactions
Glucose 6-phosphate is catalyzed by glucose 6-phosphate dehydrogenase (G6PD) in an irreversible reaction that is specific for NADP+ and its coenzyme. The PPP is regulated primarily by G6PD, and NADPH is a potent competitive inhibitor of the enzyme.
The second oxidative reaction produces a pentose sugar phosphate, ribulose 5-phosphate, and C02. This pentose can return to glycolysis through glyceraldehyde 3-phosphate or can be routed to nucleotide biosynthesis.
