Iron
last authored: June 2015, Jayneel Limbachia & Baljot Randhawa
last reviewed: Jan 2016, Dr Bhatt
Introduction
Iron is a key component in our body’s everyday processes that provide us with energy, and is required for proper immune function. Most importantly, iron keeps our oxygen levels saturated. In pregnant women, iron is required to adequately supply the placenta and the fetus with increased red blood cell mass, required for the fetus’s normal neural development.
Iron deficiency is a major contributor to illness in both developing and developed countries, and though rarer, iron toxicity can also cause great harm. Therefore, education and availability for appropriate intake are critical components of public and primary health care.
Function
The molecular element, Iron, can be found in many different states in nature. These states are known as valences, designated as +2, +3 or sometimes +4. These different states have an effect on absorption and function.
Iron has two major functions in the body:
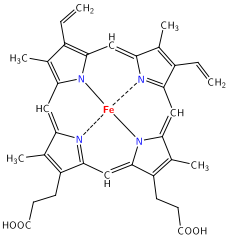
Oxygen Transport: Iron is an essential component of the porphyrin, heme. Heme is a co-factor of hemoglobin in red blood cells, which binds and transports oxygen from the lungs to all the tissues in the body. Porphyrin uses four nitrogen atoms to complex a metal cation like iron (Fe) within the center (fig 1), allowing hemoglobin to bind oxygen in the heme molecule in a stable complex. Most of the iron in the human body is found in hemoglobin. Iron is also a component of the protein myoglobin - an oxygen binding protein in the muscles that provides oxygen to our skeletal and heart muscles during metabolism.
Cofactor in biological reactions: Iron plays a role in the transfer of oxygen by being an integral part of the cytochromes and enzymes (as a cofactor) that make up the electron transport chain and the citric acid cycle—a key step in mitochondrial ATP production.
Dietary Requirements & Sources
Iron is an essential mineral that is not produced by the human body; therefore the body’s requirement for iron must be satisfied through the diet. Common dietary sources include meat, legumes, whole grains and some vegetables.
It is important to note dietary iron comes in two forms; heme and non-heme iron. Heme iron a complex of iron within the molecule protoporphyrin IX (fig 1), which can lead to better or easier absorption compared to non-heme iron (Office of Dietary Supplements, 2015). Heme iron is found only in meats, fish and poultry, while non-heme iron is also found in vegetables, grains, legumes, as well as meats.
Table 1 lists recommended amounts established by the American and Canadian Dietary Reference Intakes; these amounts are based on a diet of both vegetarian and non-vegetarian foods (Health Canada, 2010). Those with a strict vegetarian diet need to increase the recommended dietary amount, as much as 1.8 times, because of decreased bioavailability of non-heme iron (Office of Dietary Supplements, 2015).
Age |
Male (mg/day) |
Female (mg/day) |
Pregnant (mg/day) |
Lactating (mg/day) |
0- 6 months |
0.27 |
0.27 |
|
|
7-12 months |
11 |
11 |
|
|
1-3 years |
7 |
7 |
|
|
4-8 years |
10 |
10 |
|
|
9-13 years |
8 |
8 |
|
|
14-18 years |
11 |
15 |
27 |
10 |
19+ years |
8 |
18 |
27 |
9 |
Table 2 shows the amount of total iron (non-heme and/or heme) in a sample of foods (CNF, 2010). Items that have asterisks include heme iron.
Food Item (serving size) |
Amount of Iron (mg) |
Meats and Alternatives |
|
Pork liver (75g)* |
13.4 |
Pumpkin seed kernels (60 mL) |
8.5 |
Beans, baked (175 mL) |
3.7 |
Tofu, medium or firm (150g) |
2.0-7.0 |
Goat, roasted (75g)* |
2.8 |
Beef stew (250 mL)* |
2.2 |
Sardines and Shrimp (75g)* |
2 |
Duck (75g)* |
1.8-7.4 |
Eggs (2), Chicken and Lamb (75g)* |
1-1.8 |
Nuts, peanuts and sunflower seeds (60 mL) |
0.5-2 |
Fish, such as salmon, trout, halibut, haddock, perch (75g)* |
0.5-1 |
Grains |
|
Teff, Millet (125 mL), Cold Cereals (iron enriched) (30g) |
2.73-4 |
Pasta, iron enriched (125 mL) |
1-1.5 |
Bread, iron enriched (1) |
1 |
White Rice, cooked (125 mL) |
0.9 |
Vegetables and Fruits |
|
Asparagus (6 spears) |
2.1 |
Some vegetables, Pumpkins, Artichoke hearts, Peas, Potatoes, Beets, Spinach (125 mL) |
1.0-2.0 |
Raisins (60 mL) |
0.7 |
Corn, sweet, on or off cob (125 mL) |
0.5 |
Plantain, baked or boiled (125 mL) |
0.5 |
Some fruits, Apple (1), Pineapple and Watermelon, Papaya, Mango (125 mL) |
0.1-0.2 |
Milk and Alternatives |
|
Cheese, cottage – 1% M.F. (125 mL) |
0.2 |
Milk, Buttermilk and Yogurt - 1-2% M.F. (250 mL) |
0.1 |
Kefir, plain (175 mL) |
0.1 |
To maximize bioavailability, food sources that contain heme iron or are rich in Vitamin C should be consumed with non-heme iron sources to enhance absorption of non-heme iron (AIS, 2009; Iron and Iron Deficiency, 2011). More factors that increase absorption will be discussed below.
Absorption, Metabolism, Storage & Transport
Absorption Depending on the type of iron ingested, 5-35% is absorbed (Abbaspour, Hurrell, & Kelishadi, 2014). Hepcidin, a peptide hormone secreted by the liver, regulates the absorption of iron and the distribution of it throughout the body. Hepcidin can control absorption and sequestering of iron by binding to iron export channels in the gut, but also breaks down transport proteins in the cells, preventing exportation. Reduction in hepcidin will cause increase iron absorption and availability through the body and is regulated by iron levels in iron stores.
Table 3 displays factors that may increase or decrease the absorption or uptake of iron within the human body (Abbaspour, Hurrell & Kelishadi, 2014; Brown, 1963).
Factors Increasing Absorption |
Factors Decreasing Absorption |
|
|
Transport Transferrin is the major transport protein for iron in the body. Once released into plasma, iron is readily converted to the insoluble ferric state (Fe3+), requiring a transport protein like transferrin for circulation. Each transferrin molecule has two binding sites which bind iron that is released into the plasma by gut enterocytes and reticuloendothelial macrophages. Transferrin-bound iron can be internalised into cells through transferrin-receptor mediated endocytosis. All internalised iron is sent to the mitochondria for the synthesis of heme, iron-sulfur clusters and/or storage with cytosolic ferritin. The iron-sulfur clusters are crucial for proper oxidative phosphorylation to occur in the electron transport chain, while the heme synthesis is crucial for proper hemoglobin structure and oxygen binding in the blood. Ferritin or its degraded form, hemosiderin, store iron in an insoluble form which can be found largely in the liver, spleen and bone marrow of the human body (Office of Dietary Supplements, 2015). However, it should be noted that lack of ferritin or any iron regulatory proteins within intestinal cells may cause a “mucosal block”; where iron may not pass at the cellular mucosal level since there is not enough ferritin for transport through the cell and/or storage.
Storage and Major Use Because of its toxicity, free soluble iron is complexed with a group of proteins known as hemoproteins, which carry out many complicated molecule binding and sensing functions. These storage compounds include hemoglobin, myoglobin, heme enzymes or non-heme compounds like transferrin, flavin-iron enzymes and ferritin.
About two thirds of the body’s iron is found within the hemoglobin of circulating red blood cells; about 25% in other iron stores that can be mobilised easily, and the remaining 15% of iron is bound to myoglobin in the muscle and enzymes used in oxidative metabolism or other cell functions (Abbaspour, Hurrell & Kelishadi, 2014). A small amount is found in the plasma, bound to transferrin. Macrophages, found in the reticuloendothelial system (connective tissue throughout body), absorb iron from phagocytosed, or ingested, red blood cells.
Iron Deficiency
main article: iron deficiency
Iron’s association with blood means that loss of blood plays a significant role in iron deficiency. Consequently, iron losses are a source of major concern for women undergoing menses, during pregnancy, childbirth, and/or for anyone in general when faced with a severe hemorrhage due to trauma, injury, etc. Severe iron losses can lead to iron deficiency. Iron Deficiency (ID) generally follows three phases: pre-latent, latent and frank. Pre-latent phase is characterized by the depletion of tissue iron stores, latent phase is marked by low serum iron and consequences without anemia, and the frank iron deficiency involves iron deficiency anemia. Iron Deficiency Anemia (IDA) is the most commonly diagnosed form of ID. It is defined as a low hemoglobin concentration in blood. It is a severe form of ID which arises when the functional iron (in addition to the storage form of iron, which is not part of enzymatic and cellular processes) starts to deplete. In general, patients with mild ID are asymptomatic. As severity increases, signs and symptoms become more prevalent. Populations at-risk for Iron Deficiency Frequent or major blood loss, poor nutritional habits, and/or rapid growth are all factors that may cause individuals to become susceptible or at-risk for iron deficiency. The following list represents possible population groups at greater risk for iron deficiency:
- Pregnant women
- Infants and young children
- Frequent blood donors
- People with cancer (mainly colon cancer)
- People with heart failure
- Athletes
Toxicity
For the healthy adult, Canadian and American recommendations place the Tolerable Upper Limit for Iron is as follows (Health Canada, 2010):
- Infants and children (age 0-13 years) = 40 mg/day
- Males and Females (14+) = 45 mg/day
These guidelines are important as iron overdose can prove to be corrosive to the body and the prognosis may include these common signs and symptoms, often experienced in this order (Iron Ingestion Portal, 2013-14):
- Diarrhea, vomiting, abdominal pain and GI bleeding.
- Hypotension with circulatory failure, lactic acidosis with circulating iron
- Prolonged iron toxicity may lead to hepatotoxicity and prove to be fatal
Health care providers must be aware of potential iron toxicity when prescribing supplements to patients with ID or IDA. In addition, patients receiving regular blood transfusions are also susceptible to iron overload in the body, eventually leading to toxicity. Health care providers should check ferritin levels regularly on patients receiving regular transfusions.
Furthermore, hemochromatosis is a disease that occurs when too much iron accumulates in tissues and results in eventual tissue damage. Hereditary hemochromatosis is most commonly caused by a mutation in the hemochromatosis gene (HFE). Many people carry a single mutation - up to 1 out of every 10 Caucasian males. However, only 4.4 out of 1,000 people of Caucasian descent are homozygous and have consequences of this disease (Office of Dietary Supplements, 2015). People with hereditary hemochromatosis can develop signs, such as, liver cirrhosis, hepatocellular carcinoma, heart disease, and impaired pancreatic function by their thirties if not treated adequately with iron chelation or phlebotomy (Office of Dietary Supplements, 2015).
Multiple Choice Questions
What is the primary or the biggest role of iron in our body?
- as a component of hemoglobin that carries oxygenated blood
- as a component of the cell membrane
- in Protein synthesis
- in DNA synthesis
- Diarrhea and Vomiting
- Hepatotoxicity
- fatigue and general weakness
- GI bleeding
- Vitamin C
- Heme Iron
- None of the above
- A & B
- Males
- Pregnant women
- Lactating women
- Females (not impregnated or lactating)
- Fish (75g)
- Teff (cooked)
- Beans (baked)
- Plantain (baked/boiled)
What are some of the common signs and symptoms of anemia?
What helps absorption of non-heme iron?
Which individuals, aged 19+ need the most iron?
Which product has the most total iron stores?
Resources and References
Resources:
http://www.cdc.gov/nutrition/everyone/basics/vitamins/iron.html
http://drhoffman.com/article/iron-deficiency-and-toxicity-3/
http://www.hc-sc.gc.ca/fn-an/pubs/nutrition/iron-fer-eng.php
Abbaspour, N., Hurrell, R., & Kelishadi, R. (2014). Review on iron and its importance for human health. Journal of Research in Medical Sciences?: The Official Journal of Isfahan University of Medical Sciences, 19(2), 164–174. Australian Sports Commission: AIS. (2009, November 1). Retrieved May 11, 2015, from http://www.ausport.gov.au/ais/nutrition/factsheets/basics/iron_-_are_you_getting_enough
Beard, J., & Tobin, B. (2000). Iron status and exercise. The American journal of clinical nutrition, 72(2), 594s-597s. Bersamin, A., Hathaway, C., & Zidenberg-Cherr, S. (2005). Iron and Iron Deficiency Anemia. Retrieved April 28, 2015, from http://nutrition.ucdavis.edu/content/infosheets/fact-consumer-IronAndAnemia.pdf
Bhutta, Z. (2003). A situational analysis of micronutrient malnutrition among women and children in Pakistan & some preliminary programmatic recommendations. A Report for the Micronutrient Initiative & Nutrition Cell, Ministry of Health, Government of Pakistan.
BROWN, E. B. (1963). The absorption of iron. The American journal of clinical nutrition, 12(3), 205-213.
Canadian Nutrient File (CNF), 2010. (2013, July 10). Retrieved May 11, 2015, from http://www.hc-sc.gc.ca/fn-an/nutrition/fiche-nutri-data/cnf_downloads-telechargement_fcen-eng.php
Dietary Sources of Iron. (2010, January 28). Retrieved May 11, 2015, from http://www.mckinley.illinois.edu/handouts/dietary_sources_iron.html
Galy, B., Ferring-Appel, D., Becker, C., Gretz, N., Gröne, H. J., Schümann, K., & Hentze, M.W. (2013). Iron regulatory proteins control a mucosal block to intestinal iron absorption. Cell reports, 3(3), 844-857.
Harvard: The interaction of iron and erythropoietin. (2000, May 19). Retrieved May 25, 2015, from http://sickle.bwh.harvard.edu/iron_epo.html
Health Canada. (2010). Dietary Reference Intakes Tables. Retrieved from http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/nutrition/dri_tables-eng.pdf
Hegde, N., Rich, M., & Gayomali, C. (2006). The Cardiomyopathy of Iron Deficiency. Texas Heart Institute Journal, 33(3), 340-344.
Iron and Iron Deficiency. (2011, February 23). Retrieved May 11, 2015, from http://www.cdc.gov/nutrition/everyone/basics/vitamins/iron.html
Iron Ingestion Portal. (2013). In Comprehensive Advanced Life Support (Vol. 3).
Johnson, L. (2003). Essential medical physiology (3rd ed.). Amsterdam: Elsevier Academic Press.
Office of Dietary Supplements: Iron. (2015, February 19). Retrieved May 11, 2015, from http://ods.od.nih.gov/factsheets/Iron-HealthProfessional/#en17